If you are a current patient, we thank you for entrusting Orthofix with your healing process. We have compiled a list of Frequently Asked Questions (FAQ) that may be of assistance to you. Please contact us directly by phone or by email.
Yes. Bone growth stimulation produces a signal at the fusion site like the one your own body generates to induce normal bone healing. The PEMF therapy emitted by Orthofix bone growth stimulators was specially designed with your safety in mind, and is similar in strength to what you’re exposed to naturally from the magnetic field of the earth. More than 700,000 Orthofix patients have worn our stimulators to increase the probability of fusion success or to heal a non-union fracture.
Yes. Physio-Stim, Spinal-Stim and Cervical-Stim are FDA approved as a Class III medical devices. Physio-Stim was approved by the FDA in 1986, Spinal-Stim was approved by the FDA in 1990, and Cervical-Stim was approved by the FDA in 2004.
Indication
The Physio-Stim is indicated for the treatment of an established nonunion acquired secondary to trauma, excluding vertebrae and all flat bones, where the width of the nonunion defect is less than one-half the width of the bone to be treated. A nonunion is considered to be established when the fracture site shows no visibly progressive signs of healing.
Contraindication
Use of this device is contraindicated where the individual has synovial pseudarthrosis.
Warnings
Precautions
Adverse Effects
Rare instances of reversible minor discomfort have been reported. They were: cumbersome or uncomfortable, tingling or pain and minor skin rash.
Indication
Orthofix Spinal-Stim is a non-invasive electromagnetic bone growth stimulator indicated as a spinal fusion adjunct to increase the probability of fusion success AND as a nonoperative treatment for salvage of failed spinal fusion, minimum nine months postoperative.
Contraindication
Cardiac pacemakers may be adversely affected by exposure to PEMF. Use of this device is contraindicated where the individual has an implanted cardiac pacemaker.
Warnings
Precautions
Adverse Events
Rare instances of reversible minor discomfort have been reported. They were: cumbersome or uncomfortable, minor tingling or pain, minor skin rash, insomnia, fainting, nausea/diarrhea, and polymenorrhea.
Indication
The Cervical-Stim Cervical Fusion System is a noninvasive, pulsed electromagnetic bone growth stimulator indicated as an adjunct to cervical fusion surgery in patients at high risk for non-fusion.
Contraindication
There are no known contraindications for the Cervical-Stim as an adjunct to cervical spine fusion surgery.
Warnings
Precautions
Adverse Effects
Adverse effects may be experienced when using the Cervical-Stim. These adverse effects may include: increased pain, numbness and tingling, headache, migraines and nausea. These effects may or may not be directly related to the use of the Cervical-Stim. Any adverse effects that are related to the Cervical-Stim should stop when you discontinue use.
The safety of use of this device during pregnancy and nursing in humans has not been established.
The effect of PEMF treatment was studied in patients with skeletal maturity and has not been studied in patients lacking skeletal maturity. Your doctor will determine when use is medically appropriate.
Using Spinal-Stim with an implanted cardiac pacemaker or defibrillator is contraindicated, while it’s a warning with Cervical-Stim and Physio-Stim.
Demand type pacemaker operation may be adversely affected by exposure to pulsed electromagnetic fields. Physicians should not prescribe a Spinal-Stim, Cervical-Stim, or Physio-Stim for application which may place the treatment transducer in close proximity to the pacemaker. Further screening by the attending cardiologist is recommended (such as with an electrocardiogram).
It’s important to consult your cardiologist, who can run tests to determine whether the device will affect your specific pacemaker model.
After your physician determines that you would benefit from a bone growth stimulator, he or she provides Orthofix with a written prescription and other information required by your insurance provider to determine whether the device is covered under your plan. Orthofix then works with your insurer to determine coverage before you receive the device. This process can take a few days or even several weeks.
Insurance policies are different depending on the plan you have chosen. If coverage guidelines are met, the bone growth stimulator is accepted and approved by the majority of private and public health plans, including Medicare, Medicaid and workers’ compensation plans. Some plans include a deductible, co-payment, or other co-insurance amount. Please see "Is there financial responsibility for patients?" below.
Health insurers, including Medicare, typically cover only those items and services which are determined by their policy to be “reasonable and necessary” for treating specific medical conditions. To determine medical necessity, health insurers require providers such as Orthofix to provide information about your diagnosis to determine whether the device is covered by your insurance plan.
Even if an item is considered medically necessary and, therefore, covered by insurance, some health insurers require you to pay a portion of the cost. These costs could include a deductible, co-payment or other co-insurance amount. For Medicare patients, the co-insurance amount for a bone growth stimulator is generally 20% of the Medicare allowable amount. For patients with other health insurance, the co-insurance amount varies by insurer.
Yes. If your insurance has determined that you have a coinsurance/deductible, you will receive a bill with instructions for payment. Please visit orthofix.com or spinestimulation.com for details.
If this happens, the claim will be pursued by our appeals processing department on your behalf. If all appeals are exhausted and your contracted provider has denied medical necessity, you may receive a claim. If you have not already made prior payment arrangements and you receive a claim, please contact Patient Services at 1-800-535-4492 to discuss payment options and/or arrangements.
An ABN is an Advance Beneficiary Notice of Noncoverage for Medicare patients. This document gives patients advance notice that Medicare may not pay for the item prescribed by the physician for their condition. The ABN informs you of your financial responsibility if you choose to receive the device. If an ABN is required for your specific situation, you will be asked to sign it before you receive the bone growth stimulator.
Like the ABN, the Advance Notice of Noncoverage (ANN) gives non-Medicare patients advance notice that their insurer may not pay for the item prescribed by the physician for their condition. The ANN informs you of your financial responsibility if you choose to receive the device. If an ANN is required for your specific situation, you will be asked to sign it before you receive the bone growth stimulator.
Please contact Orthofix to discuss payment options. Orthofix has a patient financial assistance program for people who demonstrate financial need based on established guidelines.
Orthofix is required by law to collect a patient’s co-insurance or other amount owed for the bone growth stimulator. Under specific and limited circumstances, such as when we have verified a patient’s financial hardship, Orthofix may waive or reduce the obligation to pay an amount owed.
Patients with balances due resulting from limited or no insurance coverage may qualify for our patient financial assistance program. Upon request, Orthofix Patient Care Billing Specialists will work with you to establish a payment plan, or use established guidelines to assess your eligibility for a financial hardship waiver or reduction of the amount owed. Full or partial eligibility is determined by documented household income and family size. If eligible, patients are required to complete a financial hardship application and return the signed application to Orthofix. A determination letter is then sent by Orthofix upon receipt of the completed application.
Do not be alarmed if you receive a denial from your insurance company. You have the right to appeal!
Your health plan has an internal appeals process that includes up to two separate levels, and a third external review option. It is important to note that each review level requires additional information be submitted. This additional information may include lab reports, office notes or scientific literature not previously reviewed.
Under the Affordable Care Act, patients have the right to appeal a health insurance company’s decision to deny payment for a claim, including the denial of a claim for an Orthofix bone growth stimulator. However, it is imperative that patients check their own health plans to follow the policies and procedures applicable to appeals. Your state may have Consumer Assistance Programs that can help you file an appeal or request a review of your health insurance company’s decision if you are not sure what steps to take. Your insurance company should have provided you with information about how to file an appeal and the appeals process when you were enrolled in coverage, and there may be information about the process on the plan’s website. Visit LocalHelp.HealthCare.gov to find help in your area. For additional information, you may also call your health plan or state insurance regulator.
Appeals are most likely to go in your favor when the information is concise, complete, and details are based on fact rather than emotion. Be sure to retain original records for your files while submitting copies to the health plan for the appeal. Orthofix may have many of these documents and can assist you in compiling your Appeal packet upon request.
Getting started:
Important information to also enclose with your Appeal:
How do I follow up on my appeal?
Yes, it can be worn over an orthopedic brace or clothing without affecting the PEMF signal as it will still reach the fusion site.
You won’t feel the PEMF therapy, and the device is lightweight and adjustable for a comfortable fit. It is powered with a rechargeable battery, which allows the unit to be portable. You can sit, stand, sleep, walk, recline, and drive while using your device. With your physician’s approval, you can resume a normal activity level.
Treatment is based on a daily therapy schedule. Your doctor will prescribe the device for a certain number of hours each day, based on your needs. The minimum daily treatment time is two hours per day for Spinal-Stim, three hours per day with Physio-Stim, and four hours per day with Cervical-Stim.
Yes. You can split up treatment sessions. It is recommended that the treatment sessions be a minimum of 60 minutes. Patients have the flexibility to receive treatment at any time during the day. The device has a built-in 24 hour clock which allows treatment each day between the hours of 12:00 a.m. through 11:59 a.m. (Central Time, unless adjusted for your time zone).
No. You have the flexibility to receive your treatment at any time during the day. The device has a built-in 24 hour clock which resets daily at 12:00 midnight, Central Time, unless adjusted for your time zone. Additionally, you may choose to break your total daily prescribed treatment into a number of shorter sessions lasting at least one hour each, in accordance with your doctor’s instructions.
The healing process itself determines the duration of the treatment, and your physician will closely monitor your progress. Your individual risk factors (such as smoking, multi-level fusion, and graft type) and your compliance in wearing the device will factor into the duration of your treatment. To promote your healing, it is very important that you wear the bone growth stimulator daily as prescribed. Your doctor may require that you bring your unit in on your follow-up visits to check your compliance. Most patients wear the device between three and nine months.
Humans can’t sense a PEMF waveform, so you’ll never feel a treatment. If your device display shows a timer countdown, the unit should be working. If at any time the device stops showing a treatment timer or shows a code starting with the letter E, please contact our Patient Services at 1-800-535-4492.
One year. If at any time you need a replacement charger or foam insert cushion, please contact Patient Services at 1-800-535-4492.
Orthofix bone growth stimulators are devices that should only be used by the person for whom it is prescribed. The device is yours to keep once your treatment is complete.
While the battery for Orthofix bone growth stimulators will last for an average of ten treatment hours, it is strongly recommended that the device be recharged after completing daily treatment. The device will not deliver treatment while charging
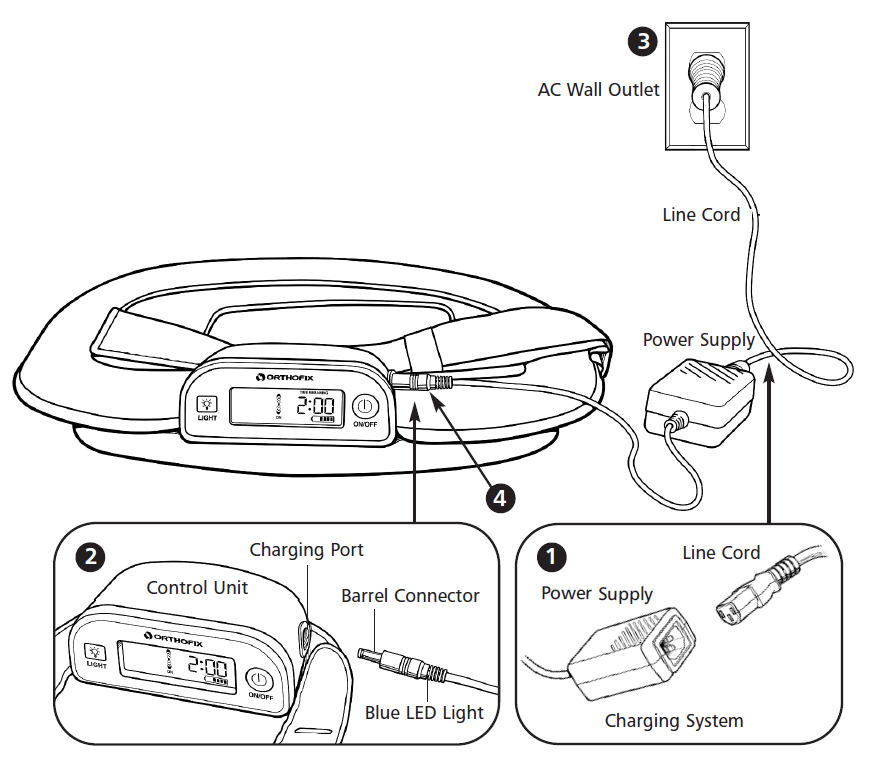
Please check that the Line Cord is plugged all the way into the AC Wall Outlet and that the power supply is fully connected. If the power supply is fully connected, the blue LED will be lit on the barrel connector. If the power supply and the stimulator are connected properly, the unit displays battery icon with scrolling bars.
Spinal-Stim, Physio-Stim and Cervical-Stim can be cleaned by wiping surfaces with a damp, soft cloth (wet with water only). Do not use solvents or expose to excessive moisture.
Cleaning foam inserts or cushions:
The unit has expired. Consult with your physician.
When traveling by air, it is recommended to pack stimulators with checked luggage. If taken onboard the airplane, it should be turned off when passing through security screening equipment, as the device could be damaged. The instruction manual should be taken with you to quickly and easily identify the device for security personnel.